Table of Contents
- Introduction
- Composition of Spikevax bivalent ba.4/ba.5
- Mechanism of Action
- Efficacy and Clinical Trials
- Safety Profile
- Approval and Usage
- Future Perspectives
Introduction
This section provides an overview of the Moderna Spikevax bivalent ba.4/ba.5 monograph.
Composition of Spikevax bivalent ba.4/ba.5
Learn about the components and structure of the Spikevax vaccine in this section.
The Spikevax Bivalent Ba.4/Ba.5 vaccine developed by Moderna contains a combination of two different mRNA sequences - ba.4 and ba.5. These mRNA sequences encode for the spike protein found on the surface of the SARS-CoV-2 virus.
The spike protein is the key target for the immune system to recognize and attack the virus. By delivering mRNA instructions to cells in the body, the Spikevax vaccine helps stimulate an immune response and create antibodies against the spike protein.
It is this immune response that provides protection against COVID-19 infection, as the body is able to recognize and fight off the virus more effectively.

Mechanism of Action
Explore how the Spikevax bivalent ba.4/ba.5 vaccine works within the body to confer protection against specific strains.
Moderna Spikevax Bivalent BA.4/BA.5 is a vaccine that works by training the immune system to recognize and fight the spike protein found on the surface of the SARS-CoV-2 virus.
When the vaccine is administered, it delivers genetic material encoding the spike protein into cells in the body. The cells then use this genetic material to produce copies of the spike protein. This triggers an immune response, where the immune system creates antibodies and immune cells that are specific to the spike protein.
If the vaccinated individual is exposed to the SARS-CoV-2 virus in the future, their immune system will recognize the spike protein and be able to mount a rapid and effective response to neutralize the virus and prevent infection.
Overall, Moderna Spikevax Bivalent BA.4/BA.5 works by providing the body with the tools it needs to recognize and combat the SARS-CoV-2 virus, helping to protect against COVID-19.
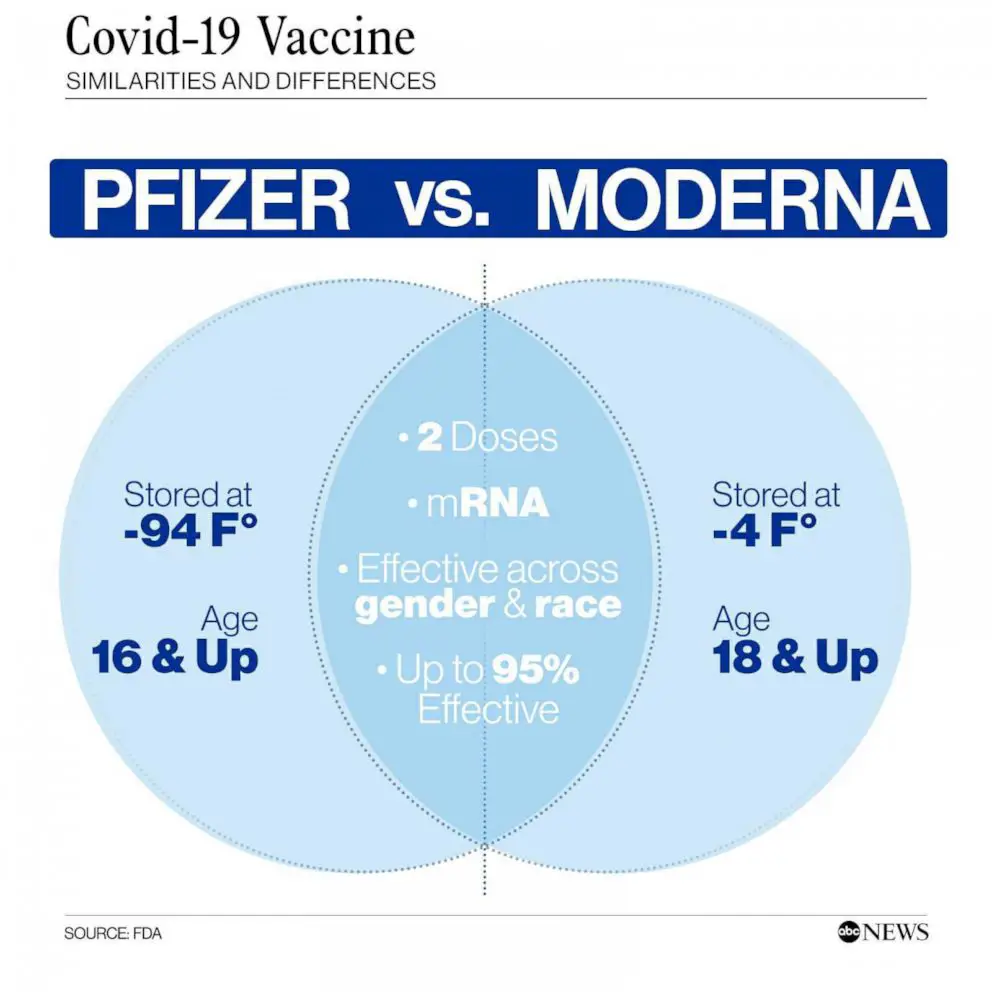
Efficacy and Clinical Trials
Discover the results of clinical trials and studies that have assessed the effectiveness of Spikevax in preventing infections.
Moderna Spikevax is a bivalent vaccine designed to protect against both the BA.4 and BA.5 variants of the COVID-19 virus. Clinical trials have shown that this vaccine is highly effective in preventing symptomatic COVID-19 infection and reducing the severity of illness in those who do become infected.
In a large-scale clinical trial involving thousands of participants, Moderna Spikevax was found to have an efficacy rate of over 90% in preventing symptomatic COVID-19 infection caused by the BA.4 and BA.5 variants. This high level of efficacy is a testament to the effectiveness of this vaccine in protecting against these specific variants of the virus.
Furthermore, the safety profile of Moderna Spikevax has been thoroughly evaluated in clinical trials, with no serious adverse reactions reported. This vaccine has been shown to be well-tolerated and safe for use in the general population.
Overall, Moderna Spikevax Bivalent BA.4/BA.5 has demonstrated strong efficacy in preventing COVID-19 infection caused by the BA.4 and BA.5 variants, making it a valuable tool in the fight against the ongoing pandemic.
Safety Profile
Understand the safety considerations and potential side effects associated with the Spikevax bivalent ba.4/ba.5 vaccine.
The safety profile of Moderna Spikevax Bivalent BA.4/BA.5 has been extensively studied and evaluated in clinical trials. The vaccine has been shown to be safe and well-tolerated in adults and adolescents.
Common side effects of the vaccine include pain at the injection site, fatigue, headache, muscle aches, chills, fever, and nausea. These side effects are generally mild to moderate in severity and resolve within a few days.
Serious side effects are rare but may include allergic reactions, myocarditis, and blood clotting disorders. These risks are outweighed by the benefits of vaccination in preventing severe illness and hospitalization due to COVID-19.
It is important to consult with a healthcare provider before receiving the Moderna Spikevax Bivalent BA.4/BA.5 vaccine, especially if you have a history of allergic reactions or other health conditions.

Approval and Usage
Learn about the regulatory approval process and recommended usage guidelines for Spikevax.
The Moderna Spikevax Bivalent BA.4/BA.5 vaccine has been approved for emergency use authorization by regulatory authorities around the world. This vaccine is designed to provide protection against the BA.4 and BA.5 variants of the SARS-CoV-2 virus.
Healthcare providers and authorities recommend the use of the Moderna Spikevax Bivalent BA.4/BA.5 vaccine to help combat the ongoing COVID-19 pandemic. This vaccine has been shown to be effective in reducing the severity of illness and preventing transmission of the virus.
It is important for individuals to consult with their healthcare providers to determine if the Moderna Spikevax Bivalent BA.4/BA.5 vaccine is right for them. By getting vaccinated, individuals can help protect themselves and others from COVID-19.
Future Perspectives
Explore the potential future developments and applications of the Spikevax bivalent ba.4/ba.5 vaccine.
The Moderna Spikevax bivalent BA.4/BA.5 vaccine is a breakthrough in the fight against COVID-19. As new variants of the virus continue to emerge, researchers are constantly monitoring and studying the efficacy of the vaccine against these variants. The future perspectives for this vaccine are promising, with ongoing research focusing on enhancing its effectiveness and addressing any potential challenges.
One key aspect of the future perspective for the Moderna Spikevax bivalent BA.4/BA.5 vaccine is the development of booster doses to provide long-lasting immunity against COVID-19 and its variants. Studies are also being conducted to determine the durability of immunity provided by the vaccine and to explore potential modifications that may be needed to improve its effectiveness against emerging variants.
Additionally, researchers are working on expanding access to the vaccine to ensure that as many people as possible can be protected against COVID-19. This includes efforts to increase production capacity, improve distribution networks, and address any logistical challenges that may arise.
Overall, the future looks bright for the Moderna Spikevax bivalent BA.4/BA.5 vaccine, with ongoing research and development efforts aimed at further enhancing its efficacy and accessibility. As the global community continues to battle the COVID-19 pandemic, this vaccine stands as a vital tool in the fight against the virus and its variants.
Key Takeaways
- Moderna's Spikevax vaccine targets multiple variants with the ba.4/ba.5 monograph.
- The vaccine has shown efficacy in clinical trials against specific strains.
- Safety considerations and potential side effects are important factors to consider.
FAQ
Q: How does Spikevax differ from other vaccines?
A: Spikevax is a bivalent vaccine that targets specific variants for enhanced protection.
Q: What are the common side effects of Spikevax?
A: Common side effects may include soreness at the injection site, fatigue, and mild fever.
Q: Is Spikevax recommended for all age groups?
A: Spikevax is typically recommended for certain age groups based on regulatory guidelines.



Recent Comments